PDX
PDX models are now the gold standard for preclinical evaluation of innovative drugs and biomarker discovery. As pioneer in the development of those models, XenTech has developed a unique know-how associated to its models that allow providing the best added value studies.
Patient-derived tumor xenograft panels enhance the power of preclinical assays in numerous ways.
01PDX features
Faithfully
reproducing the biological features of human cancers in term of histology, genomics and proteomics
Providing
established concordance between patient and xenograft drug responses
Covering
molecular diversity of clinical disease
Allowing
identification of responsive cancer types
Allowing
biomarker exploratory studies for subsequent patient stratification

03Our collection
-
Cancer typeSubtypes
-
BreastTNBC, HER2+, ER+, PR+
-
Colorectal
-
LungNSCLC, SCLC
-
ProstatePrimary hormone-dependent, Hormone-independent variant
-
BrainGlioblastoma
-
KidneyRenal Cell Carcinoma (RCC)
-
Pancreas
-
SkinMelanoma, Merkel cell carcinoma
-
Ovary
-
Endometrium
-
Liposarcoma
-
Pediatric liver tumorsHepatoblastoma, Rhabdoid tumor
03PDX Database content
Molecular database
- Gene sequences (NGS Illumina HiSeq technology)
- Manually currated gene mutation data
- Gene expression profiles (U133plus Affy)
- Copy number variarion data (SNP6.0 Affy)
- MiRNA expression data (miRNA array)
- Protein expression (markers)
Drug response data
- PDX responses to conventional treatments
Patient data (on case-by-case basis)
- Histology
- Treatment before surgery
- Disease stage
PDX Models
Our PDX models are obtained from surgical samples grafted subcutaneously into immunodeficient mice and maintained by serial transplantations. Each new PDX model is considered as immortalized and stable after 3 passages.
A frozen master stock is made at low passages.

01MATCH-R
Thanks to the MATCH-R precision medicine trail led by Gustave Roussy, XenTech offers a growing panel of PDX established from patients who proved demonstrated acquired resistance to last generation therapies (only newly approved or in clinical evaluation drugs).
- The MATCH-R precision medicine trial led by Gustave Roussy Hospital (NCT02517892) will enroll 600 patients treated with targeted therapies in phase I/II studies between 2014 and 2020.
- Biopsies from tumor lesions are performed in patients with tumor progression under treatment following initial response. To explore the underlying resistance mechanisms, RNAseq, WES and CGH data are generated from the biopsies.
- Biopsies from 300 patients at resistance are used to generate PDX models that will be pharmacologically and molecularly characterized to verify that tumor resistance and genetic alterations observed in the patients are maintained.
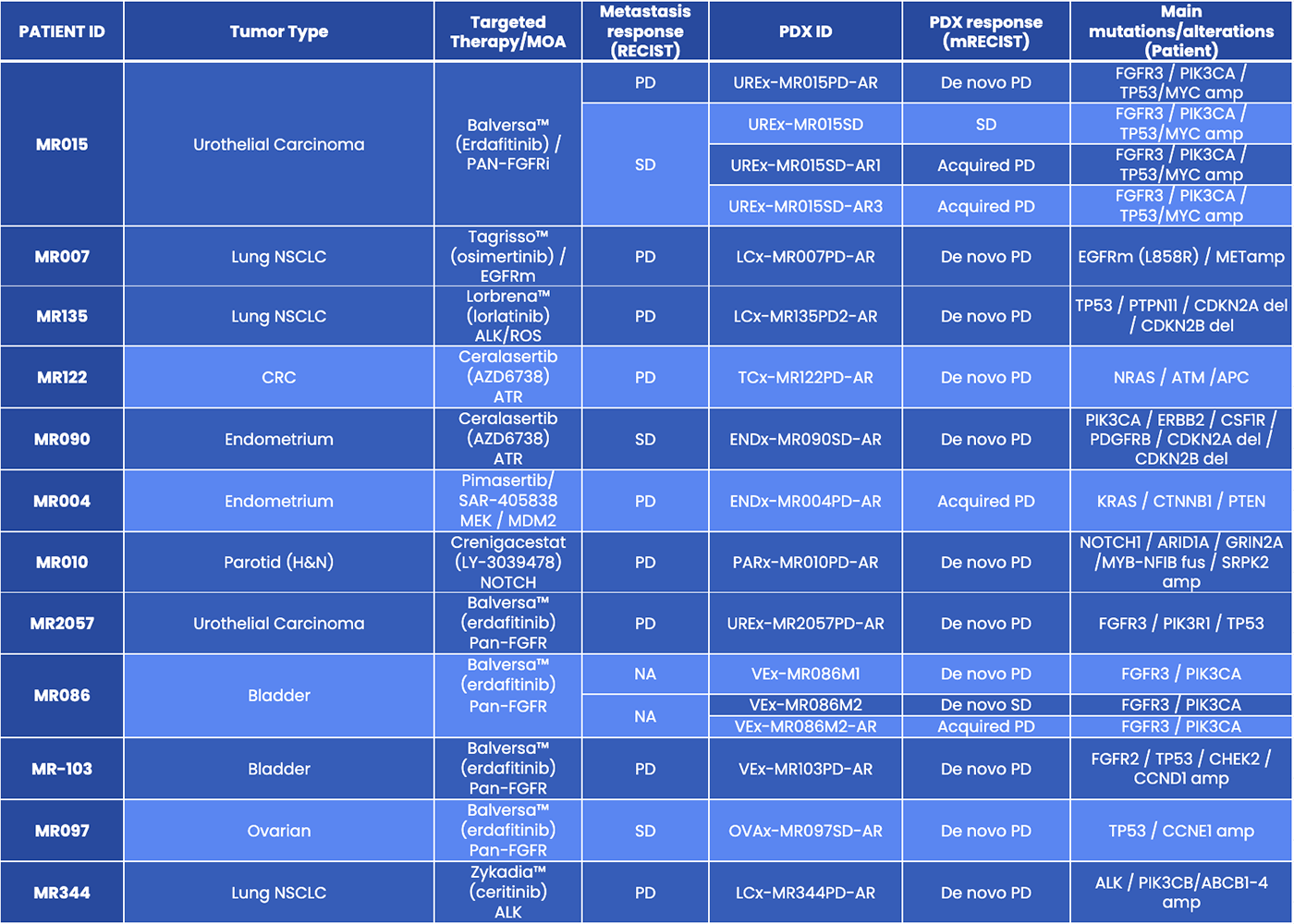
01The MATCH-R collection
01Breast cancer
XenTech offers the reference panel of Breast cancer derived PDX including
- 27 Triple negative breast cancer
- 6 ER/PR +
- 5 Her2 amplified
Those models have been extensively characterized regarding their molecular feature (WES and RNAseq, aCGH, …) as well as for response to standards of care (A/C, Capcitabline, Docetaxel, Trastuzumab, Lapatinib, PARPi, …)
01Paediatric
Supported by the European consortium ‘Innovative Medicines Initiative’ (IMI2), XenTech actively participate to the ITCC-P4 (Innovative Therapies for Children with Cancer Paediatric Preclinical Proof-of-concept Platform) project aiming at :
- Establishment and molecular characterisation of a collection of 400 PDX models of the most common pediatric solid high-risk entities
- Performing mouse trial assays using a panel of standard of care (SOC) and investigational drugs